Regenerative Medicine Market Size Worth USD 1,69,586 Mn by 2034 Driven by Stem Cells, Innovation, and Investment in New Treatments
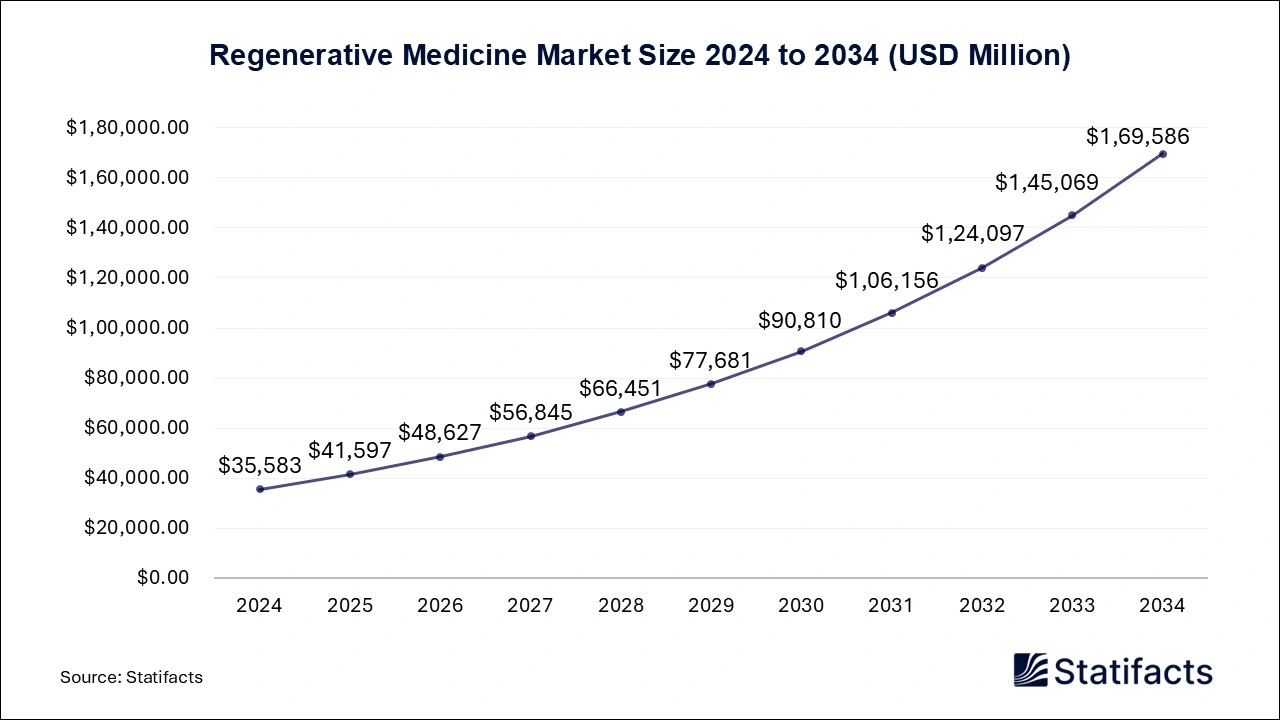
The global regenerative medicine market is expected to grow significantly, reaching USD 1,69,586 million by 2034, up from USD 41,597 million in 2025, with a robust CAGR of 16.9% from 2025 to 2034. Asia Pacific is emerging as the fastest-growing region in this sector, as highlighted in a study published by Statifacts, a sister firm of Precedence Research.
Ottawa, Sept. 17, 2025 (GLOBE NEWSWIRE) -- According to Statifacts, the global regenerative medicine market size surpassed USD 35,583 million in 2024 and is projected to hit approximately USD 1,69,586 million by 2034, growing at a CAGR of 16.9% during the forecast period from 2025 to 2034. Strong product of potential therapies, high adoption of stem cell technology in disease treatment, and increasing investment in the development of innovative medicines are driving the growth of the market.
This report is readily available for immediate delivery. We can review it with you in a meeting to ensure data reliability and quality for decision-making.
Try Before You Buy – Get the Sample Report@ https://www.statifacts.com/stats/databook-download/8139
Regenerative Medicine Market Highlights
- North America dominated the regenerative medicine market in 2024, holding the largest market share.
- Asia-Pacific is projected to experience the fastest growth rate in the regenerative medicine market from 2025 to 2034.
- The therapeutics segment dominated the market by product, accounting for the largest share in 2024.
- The banks segment is anticipated to be the fastest-growing segment by product during the forecast period.
- By therapeutic segment, oncology dominated the market in 2024, capturing the largest share.
- The cardiovascular segment is expected to grow at the fastest rate within the therapeutic category during the forecast period.
Market Overview and Industry Potential
The regenerative medicine market refers to the production, distribution, and use of regenerative medicine, which deals with the process of replacing, engineering, or regenerating human or animal cells, tissues, or organs to restore or establish normal function. Regenerative medicine seeks to replace tissue or organs that have been damaged by age, disease, trauma, or congenital issues. Regenerative medicine (RM) implies the replacement or regeneration of human cells, tissue, or organs to restore or establish normal function.
According to a report published in May 2025, the enrollment of the first patient in its clinical trials evaluating the safety and efficacy of NeuroSpan Bridge, an innovative investigational device designed to improve nerve regeneration, was announced by Auxilium Biotechnologies, a leader in regenerative medicine. This trial represents a significant step forward in the company’s mission to restore lost function for patients who suffer from debilitating nerve injuries to the extremities, including trauma like work-related injuries and car accidents. Source: BusinessWire
The benefits of using artificial intelligence (AI) in regenerative medicine are its ability to help identify the best cells for a particular patient. AI allows the development of personalized predictive models by analyzing patient data, like metabolomics, genomics, and proteomics. It helps identify the individual disease process differences that can be used to create personalized predictive models. AI informs the translation of regenerative technologies, improving clinical assessment of regenerative therapy outcomes. AI can enhance the drug discovery process of regenerative medicine by tackling the main risk associated with this process, the time taken, and the high costs.
Case Study: Tulsi Therapeutics’ Tulsi-28X – A Breakthrough in Liver Regeneration
Overview
Chronic liver failure remains one of the most challenging conditions worldwide, with limited treatment options and high mortality rates. While liver transplantation is often the only cure, it is costly, complex, and not accessible to most patients. In this context, regenerative medicine is opening new frontiers one of the most promising examples being Tulsi-28X, developed by Hyderabad-based Tulsi Therapeutics.
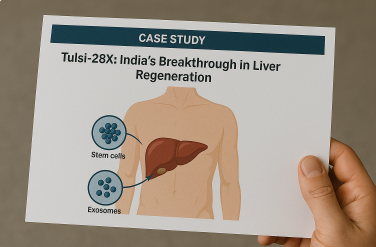
The Breakthrough
Tulsi Therapeutics, incubated at the University of Hyderabad’s ASPIRE-BioNEST, has created Tulsi-28X, a novel therapy that combines stem cells and exosomes derived from Wharton’s Jelly. This innovative approach aims to reverse liver damage and restore function, offering a potential alternative to transplants.
Key Results:
- In preclinical trials on rats, Tulsi-28X achieved zero mortality in treated groups versus high mortality in untreated ones.
- The therapy showed reversal of liver fibrosis, a major advancement in regenerative liver care.
- Tulsi has filed with India’s CDSCO to begin human clinical trials, signaling a step toward real-world application.
Global Collaboration & Future Potential
The project is being advanced in collaboration with Indiana University (USA) and PGIMER, Chandigarh, highlighting the growing international partnerships in regenerative medicine. If approved, Tulsi-28X could provide an affordable and scalable solution, especially for patients in emerging markets where transplants are financially out of reach.
Why This Matters for the Market
- Asia-Pacific Leadership: This aligns with your press release’s finding that Asia-Pacific is the fastest-growing region in regenerative medicine.
- Policy Alignment: It reflects India’s BioE3 policy push, designed to promote regenerative medicine R&D.
-
Innovation in Action: Demonstrates how startups are translating lab-based innovations into life-saving therapies.
What are the Key Recent Government Initiatives in Regenerative Medicine (Globally)?
As of 2025, more than 80 RMAT designations have been granted by the U.S. FDA and 30+ conditional approvals in Japan and Europe, reflecting increasing regulatory momentum.
-
NIH’s Regenerative Medicine Innovation Project (RMIP) — United States
This is a trans-NIH effort, set up under the 21st Century Cures Act, to accelerate clinical research on adult stem cells while ensuring safety and rigor in product characterization and trials. Source: National Institutes of Health (NIH)
-
Japan’s Regenerative CDMO Subsidy Program
The Japanese government (Ministry of Economy, Trade, and Industry) selected JCR Pharmaceuticals in 2025 for a subsidy for facility upgrades, equipment installation, and workforce training to expand biomanufacturing capacity for regenerative, cell, and gene therapies. Source: BioSpace
-
Taiwan’s Regenerative Medicine Act & Regenerative Medical Product Act
Passed in June 2024, these laws set up a legal framework in Taiwan to regulate regenerative medical technologies and products. They introduce conditional and time-limited approval pathways, safety oversight, and requirements for long-term studies.
-
BioE3 Policy in India
Under BioE3 (Biotechnology for Economy, Environment, and Employment), approved in August 2024, the government is promoting high-performance biomanufacturing, R&D in biotechnology (including stem cell / regenerative medicine), and linking it to employment and economic growth. Institutions like inStem (iBRIC-inStem, Bengaluru) are key beneficiaries of this policy. Source: inStem
-
South Korea’s Act on Advanced Regenerative Medicine & Advanced Biological Products (ARMAB)
ARMAB, first enacted in 2019 and implemented since ~2020, is being expanded in terms of patient access and regulatory frameworks. As of early 2025, the law enables access to certain regenerative medicine therapies for severe, rare, or incurable conditions that have shown safety/efficacy in clinical research. Source: PMC+1
Key Market Trends
- Favorable Regulatory Support & Fast-Track Approvals: Regulatory agencies (e.g., FDA, EMA, Japan’s PMDA) are introducing pathways like RMAT and conditional approval. These help companies bring promising therapies to patients faster.
- Shift Toward Personalized & Off-the-Shelf Therapies: Autologous (patient-specific) treatments are growing alongside scalable allogeneic therapies. This trend balances personalization with the need for cost-effective mass production.
- Rising Investments & Industry Collaborations: Public and private funding is accelerating, especially in biomanufacturing and clinical trials. Collaborations between academia, biotech, and pharma are key to scaling innovations globally.
- Integration of AI and Digital Technologies: AI and data analytics are enhancing regenerative medicine by optimizing cell therapy design, imaging, and diagnostics. Digital tools are also improving manufacturing efficiency and trial outcomes.
-
Expanding Applications Across Disease Areas: Regenerative therapies are expanding beyond orthopedics and wound care into neurology, cardiology, and ophthalmology. This diversification is driving broader adoption and revenue growth.
Customize This Study as Per Your Requirement@ https://www.statifacts.com/stats/customization/8139
Regenerative Medicine Market Dynamics
Market Drivers
- Increasing investment in the development of innovative medicines: Innovative medicines are drugs that offer new treatments or cures for conditions that were previously untreatable or difficult to treat. Drug development is critical for advancing healthcare by discovering and producing new medications to treat diseases and conditions. It drives innovation in medical technology, leading to enhancements in drug delivery systems, diagnostic tools, and medical devices.
- High use of stem cell technology in disease treatment: Stem cell technology is unique in that it possesses both diagnostic and therapeutic potential. Stem cells or bone marrow transplant replace damaged blood cells with healthy ones. It can be used to treat conditions affecting the blood cells, like lymphoma and leukemia. Stem cells are used to test new drugs for safety and effectiveness, research how diseases occur, or why specific cells develop into cancer cells, research causes of genetic defects in cells, correct parts of organs that don’t work properly, and grow new cells in a laboratory to replace damaged organs or tissues.
Restraint
-
Ethical concerns: One of the main challenges in regenerative medicine is the ethical concerns surrounding the use of embryonic stem cells. Embryonic stem cells are derived from human embryos, raising ethical questions about the destruction of embryos for research purposes. Regenerative medicine therapies have not been approved for the treatment of any orthopedic condition, such as shoulder pain, neck pain, knee pain, hip pain, back pain, tennis elbow, disc disease, tendonitis, or osteoarthritis. Risk associated with regenerative medicine technologies includes immunogenicity, tumorigenicity, and the risks associated with the implantation procedure.
Opportunity
-
Expanding use of 3D printing technology: Using 3D printing technologies, native tissue mimics can be created using biomaterials and living cells. 3D printing in healthcare is the ability to produce complex structures. This may be useful for creating models of organs or body parts for surgical planning. It can also be used to create implants and prosthetics that are more complex than what can be made with traditional manufacturing methods. As compared to traditional preparation technologies, 3D printing offers flexibility in the design of complex 3D structures within drugs, the adjustment of drug doses and combinations, rapid manufacturing, and prototyping.
Ready to Dive Deeper? Visit Here to Buy Databook & In-depth Report Now@ https://www.statifacts.com/order-databook/8139
Regenerative Medicine Market Scope
| Report Attribute | Key Statistics | |
| Market Size in 2024 | USD 35,583 Million | |
| Market Size in 2025 | USD 41,597 Million | |
| Market Size in 2028 | USD 66,451 Million | |
| Market Size in 2032 | USD 1,24,097 Million | |
| Market Size by 2034 | USD 1,69,586 Million | |
| CAGR 2025-2034 | 16.9% | |
| Leading Region in 2024 | North America | |
| Fastest Growing Region | Asia-Pacific | |
| Base Year | 2024 | |
| Forecast Period | 2025 to 2034 | |
| Segments Covered | By Product, By Therapeutic Category, and By Region | |
| Regional analysis | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa | |
| Leading Players | AstraZeneca plc, F. Hoffmann-La Roche Ltd., Integra Lifesciences Corp., Astellas Pharma Inc., Cook Biotech Inc., Bayer AG, Pfizer Inc., Merck KGaA, Abbott, Vericel Corp., Novartis AG, GlaxoSmithKline (GSK) and Other. | |
Kindly use the following link to access our scheduled meeting@ https://www.statifacts.com/schedule-meeting
Regenerative Medicine Market Segmentation
Product Insights
The therapeutics segment dominated the regenerative medicine market in 2024. Regenerative medicine has the potential to heal or replace tissues or organs damaged by age, disease, or trauma, and also to normalize congenital defects. The pharmaceutical industry has been slow to adopt new technologies in stem cell biology and regenerative medicine. Therapeutic reprogramming represents a transformative paradigm in regenerative medicine, developing new approaches in gene therapy, biologics, small molecule drugs, and cell therapy to address unmet medical challenges.
The banks segment is observed to be the fastest-growing in the market during the forecast period. Regenerative medicine banking is the process of collecting, processing, and storing potentially life-saving stem cells for future use in regenerative medicine and therapies. To support transplant needs and help make improvements in regenerative medicine, tissue banks are essential parts of the modern medical system.
Therapeutic Category Insights
The oncology segment led the market in 2024 due to the regenerative medicine therapy, which holds the promise of more natural and aesthetic functional tissue. Regenerative therapy helps to improve treatment outcomes for specific blood cancers. Regenerative therapy, also known as stem cell therapy, promotes the repair response of diseased, dysfunctional, or injured tissue using stem cells or their derivatives. Regenerative medicine use in oncology includes improving the body’s immune response, reducing side effects, and restoring tissue function after cancer treatment to fight cancer cells.
The cardiovascular segment is expected to be the fastest-growing in the market during the forecast period. Because, as compared to traditional treatments that manage symptoms, regenerative medicine focuses on repairing the damaged heart muscle itself. This offers the potential to reverse some of the damage caused by heart disease, leading to better long-term outcomes for patients. Cellular regenerative therapies using many stem cells to improve the functional recovery of the heart, mainly by cytokine paracrine effects.
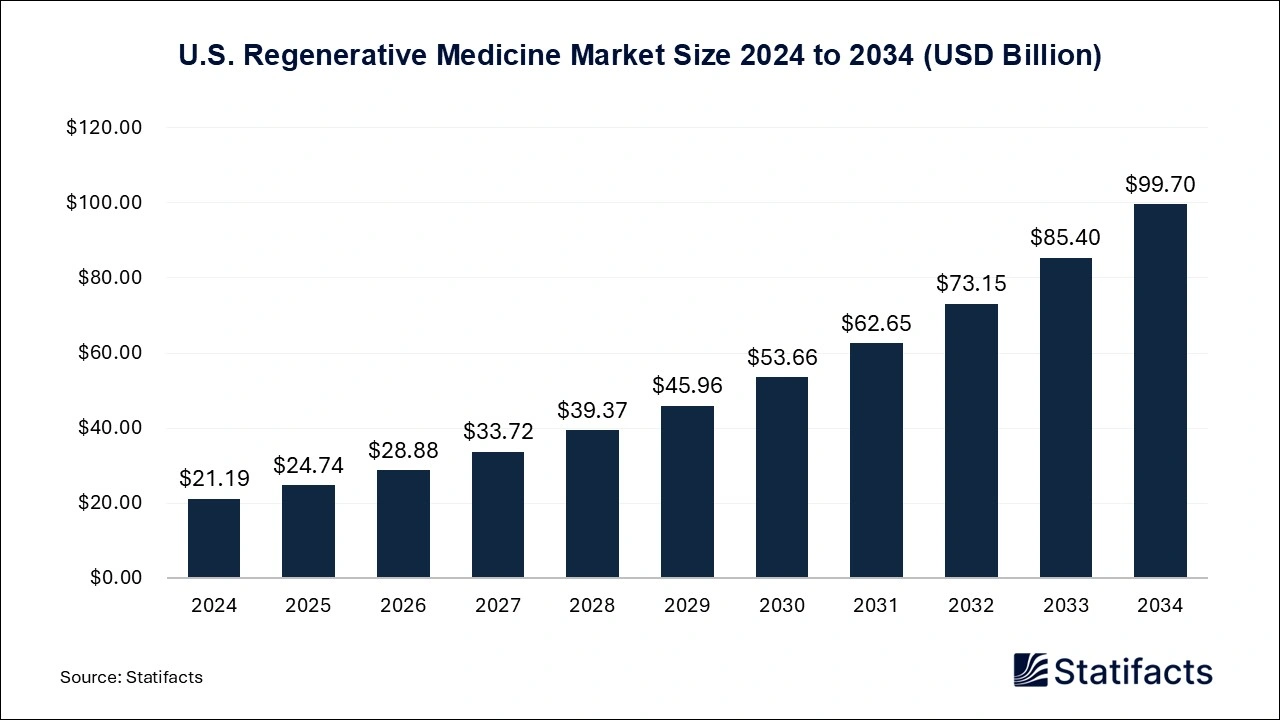
U.S. Regenerative Medicine Market Size 2025 to 2034 (USD Billion)
The U.S. regenerative medicine market size was exhibited at USD 24.74 billion in 2025 and is projected to hit around USD 99.70 billion by 2034, growing at a CAGR of 16.75% during the forecast period 2025 to 2034.
North America dominated the regenerative medicine market in 2024. Due to the rising prevalence of chronic diseases, increasing investment in innovative medicines development, and the rising senior population, the market in the North American region is growing. The region’s strong focus on innovations in gene and cell therapies and funding for research and development, enabling market growth. Additionally, regulatory advancements like the FDA’s RMAT program are further adding innovations and approvals for the novel regenerative medicine across North America.
The United States is the leader in innovation in regenerative medicines. The stem cell-based treatment that is routinely reviewed and approved by the U.S. Food and Drug Administration (FDA) is hematopoietic (or blood) stem cell transplantation. It is used to treat patients with cancers and diseases that affect the blood and immune system. Key players include a mixture of biotech companies, academic and research institutions, hospitals, venture capital investors, and governmental agencies, which contribute to the country's market.
- In August 2025, the launch of the Second National Television Campaign showcasing U.S.-based regenerative stem cell therapies was launched by Adia Nutrition Inc., a publicly traded leader in regenerative medicine and personalized healthcare. These therapies are designed for a wide range of conditions, including advanced wound repair, chronic pain, orthopedic care, and sports injuries. Source: Nasdaq
Asia Pacific is expected to be the fastest-growing region in the market during the forecast period because the Asia Pacific region is active in regenerative medicine research, mainly in gene therapy, cell therapy, and tissue engineering. Asia has experienced significant growth in healthcare spending, driven by strong economic growth in countries like China, India, and Japan. The growing prevalence of chronic disease has boosted a spectacular shift toward regenerative medicine.
Rising chronic diseases, an aging population, and a focus on precision medicine are mainly seen in countries like China and Japan. China is the best country in the world for an emerging powerhouse in regenerative medicine. The Chinese government has made regenerative medicine a priority in its national plans (such as the Five-Year Plans), with significant budget allocations for stem cell research, tissue engineering, etc Japan is the best country in the world for fast-tracking regenerative medicine. Japan adopted laws in 2014 (Pharmaceuticals and Medical Devices Act; Act on the Safety of Regenerative Medicine) that created an explicit regulatory pathway for “regenerative medicine (cellular and tissue-based) products.”
Browse More Research Reports:
- The global nuclear medicine information management system market, valued at USD 145 million in 2024, is projected to reach USD 220.91 million by 2034, growing at a 4.3% CAGR as healthcare providers adopt advanced digital diagnostic tools.
- The U.S. concierge medicine market size was estimated at USD 8.14 billion in 2024 and is projected to be worth around USD 17.16 billion by 2034, growing at a CAGR of 7.58% from 2025 to 2034.
- The global concierge medicine market size was evaluated at USD 20.4 billion in 2024 and is expected to grow around USD 43.78 billion by 2034, registering a CAGR of 7.77% from 2025 to 2034.
- The global personalized medicine market size surpassed USD 614.24 billion in 2024 and is predicted to reach around USD 1,350.86 billion by 2034, registering a CAGR of 8.2% from 2025 to 2034.
- The U.S. precision medicine market size surpassed USD 49.81 billion in 2024 and is predicted to reach around USD 235.37 billion by 2034, registering a CAGR of 16.8% from 2025 to 2034.
- The U.S. personalized medicine market size is worth around USD 345.56 billion in 2025 and is anticipated to reach around USD 705.88 billion by 2034, growing at a notable CAGR of 8.26% from 2024 to 2034.
- The global therapeutic nuclear medicines market size accounted for USD 1,380 million in 2024 and is expected to exceed around USD 2,080 million by 2034, growing at a CAGR of 4.2% from 2024 to 2034.
- The telemedicine equipment market size is predicted to gain around USD 51,498 million by 2034 from USD 11,980 million in 2024 with a CAGR of 15.7%.
- The pediatric medicines market valued at USD 111.34 billion in 2024 and projected to reach USD 168.01 billion by 2034, growing at a CAGR of 4.2%.
- The plasma-derived medicine market valued at USD 35.21 billion in 2024 and projected to reach USD 73.24 billion by 2034, growing at a CAGR of 7.6%.
Ready to Dive Deeper? Visit Here to Buy Databook & In-depth Report Now@ https://www.statifacts.com/order-databook/8139
Regenerative Medicine Market Top Companies

- AstraZeneca Plc – AstraZeneca is investing in regenerative medicine through its research in cell and gene therapies aimed at tissue repair and chronic disease treatment.
- F. Hoffmann-La Roche Ltd. – Roche focuses on regenerative approaches by developing advanced biologics and cell-based therapies to support tissue regeneration and healing.
- Integra Lifesciences Corp. – Integra Lifesciences offers a range of regenerative products, including dermal regeneration templates and nerve repair technologies.
- Astellas Pharma, Inc. – Astellas is actively advancing regenerative medicine through its subsidiary, AIRM, which develops stem cell therapies for conditions like age-related macular degeneration.
- Cook Biotech, Inc. – Cook Biotech specializes in regenerative biomaterials derived from extracellular matrix (ECM) for surgical and wound healing applications.
- Bayer AG – Bayer is expanding into regenerative medicine by collaborating on gene and cell therapies, particularly in cardiovascular and neurological disorders.
- Pfizer, Inc. – Pfizer supports regenerative medicine through partnerships and R&D in gene therapy and tissue engineering for degenerative diseases.
- Merck KGaA – Merck KGaA engages in regenerative medicine by providing tools and technologies for stem cell research and regenerative drug development.
- Abbott – Abbott contributes to regenerative healthcare through innovations in cardiovascular tissue repair and biomaterials.
- Vericel Corp. – Vericel specializes in autologous cell therapies for the repair of cartilage, skin, and severe burns.
- Novartis AG – Novartis has entered the regenerative medicine space with investments in gene therapies and cell-based solutions for rare and chronic conditions.
- GlaxoSmithKline (GSK) – GSK is pursuing regenerative medicine through its work on genetic and cell therapies to restore function in damaged tissues and organs.
Recent Developments
- In July 2025, the launch of “J SOLUTIONS PHARMA CELLS,” a specialized cell transport service designed to support advancements in regenerative medicine, a rapidly evolving and critical medical field, was announced by Japan Airlines Co., Ltd. The rapid growth of regenerative medicine has led to a rising demand for highly specialized cell transport solutions. Source: Payload Asia
- In December 2024, the development of regenerative medicine and cell therapy by forming a joint venture was launched by Sumitomo Chemical Co., Ltd. and Sumitomo Pharma Co., Ltd. The new entity, RACTHERA Co., Ltd, will focus on the research and development of these innovative therapies within the Sumitomo Chemical Group. This initiative aims to transform the company’s technological expertise in sectors such as ICT, food, healthcare, and the environment, addressing critical societal risks and establishing robust businesses, similar to its success in ICT and agro-solutions. Source: Chemanalyst News
Segment Covered in the Report
By Product
- Therapeutics
- Primary cell-based therapeutics
- Dermatology
- Musculoskeletal
- Surgical
- Dental
- Others
- Stem Cell & Progenitor Cell-based therapeutics
- Autologous
- Allogenic
- Others
- Cell-based Immunotherapies
- Gene Therapies
- Primary cell-based therapeutics
- Tools
- Banks
- Services
By Therapeutic Category
- Dermatology
- Musculoskeletal
- Immunology & Inflammation
- Oncology
- Cardiovascular
- Ophthalmology
- Others
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
You can place an order or ask any questions, please feel free to contact us at sales@statifacts.com
Statifacts offers subscription services for data and analytics insights. This page provides options to explore and purchase a subscription tailored to your needs, granting access to valuable statistical resources and tools. Access here - https://www.statifacts.com/get-a-subscription
Contact US
- Ballindamm 22, 20095 Hamburg, Germany
- Web: https://www.statifacts.com/
-
Europe: +44 7383 092 044
About US
Statifacts is a leading provider of comprehensive market research and analytics services, offering over 1,000,000 market and custoer data sets across various industries. Their platform enables businesses to make informed strategic decisions by providing full access to statistics, downloadable in formats such as XLS, PDF, and PNG.
Our Trusted Data Partners:
Precedence Research | Towards Healthcare | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Dental | Towards EV Solutions | Nova One Advisor
Explore More Reports:
- Shore Power Market Size - https://www.statifacts.com/outlook/shore-power-market
- Ocean Current Energy Conversion Market Size - https://www.statifacts.com/outlook/ocean-current-energy-conversion-market
- Transition Piece (TP) Market Size - https://www.statifacts.com/outlook/transition-piece-market
- Aviation Power Battery System Market Size - https://www.statifacts.com/outlook/aviation-power-battery-system-market
- Aftermarket Automotive Sport Seats Market Size - https://www.statifacts.com/outlook/aftermarket-automotive-sport-seats-market
- Battery Electric Vehicle (BEV) Charging Inlets Market Size - https://www.statifacts.com/outlook/battery-electric-vehicle-charging-inlets-market
- Electric Vehicle Steering System Market Size - https://www.statifacts.com/outlook/electric-vehicle-steering-system-market
- Pumper Fire Truck Market Size - https://www.statifacts.com/outlook/pumper-fire-truck-market
- Automotive Sports Style Seats Market - https://www.statifacts.com/outlook/automotive-sports-style-seats-market
- Automotive Seat Foam Market Size - https://www.statifacts.com/outlook/automotive-seat-foam-market

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.